Abstract
Introduction: Asciminib, a first-in-class BCR::ABL1 inhibitor that works by specifically targeting the ABL myristoyl pocket (STAMP), has been recently approved by the FDA for adult patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase (Ph+ CML-CP) treated with ≥2 prior tyrosine kinase inhibitors (TKIs), as well as adult patients with Ph+ CML-CP with the T315I mutation.
The recommended asciminib doses for patients with Ph+ CML-CP without the T315I mutation are 40 mg twice daily (BID), which is the dose tested in the pivotal phase III study (ASCEMBL), and a more patient-centric dose of 80 mg once daily (QD). For patients with the T315I mutation, the approved dose of asciminib is 200 mg BID. All asciminib drug-drug interaction (DDI) and hepatic and renal impairment studies were conducted at single or multiple doses of 40 mg.
Here, we aim to assess the clinical impact of hepatic and renal impairment and of DDIs on the pharmacokinetics (PK) of asciminib and provide recommendations for eventual necessary dose adjustments.
Methods: Organ impairment effect was quantified using population pharmacokinetics (PopPK), while DDIs were predicted using physiologically-based pharmacokinetics (PBPK).
A 2-compartment PopPK model with a delayed first-order absorption and first-order elimination was developed for asciminib (Li et al. Clin Pharmacokinet, 2022). The original analysis included pooled PK data from patients receiving asciminib at 10−200 mg BID (in the phase I dose-finding study, NCT02081378) or 40 mg BID (in the phase III study ASCEMBL, NCT03106779), and identified baseline absolute glomerular filtration rate (aGFR), nominal total daily dose, body weight and formulation as significant covariates affecting asciminib PK. The model has been further extended to describe patients with renal (based on aGFR) or hepatic (based on Child-Pugh class) impairment, using data from two phase I PK studies in patients with renal (NCT03605277) or hepatic (NCT02857868) impairment.
A PBPK model was developed and validated for asciminib using SimCYP v19.1. A stepwise "middle out” approach was used leveraging in vitro, in silico and in vivo data. The PBPK model was validated using single and multiple dose PK at 20‒400 mg daily dose as well as clinical DDI and PK data in patients with organ impairment. Comprehensive DDI scenarios and PK in patients with hepatic and renal impairment, which had not been clinically tested, were simulated at the different recommended doses of asciminib (40 mg BID, 80 mg QD, 200 mg BID).
Results: There was no significant impact of hepatic function on asciminib exposure; a non-significant trend to lower clearance was observed in patients with severe hepatic impairment. The PopPK derived steady-state exposures (measured as area under the curve [AUC]0-24h) for patients with severe hepatic impairment were almost identical for both dosing regimens (40 mg BID: 13,018 ng*h/mL, 80 mg QD: 13,020 ng*h/mL), and only marginally higher than those of patients with normal hepatic function (12,638 ng*h/mL and 12,646 ng*h/mL, respectively), well within the therapeutic window of asciminib.
Renal function assessment showed that clearance was slightly decreased in patients with renal impairment, although this was not clinically relevant. For both 80 mg QD and 40 mg BID, the median predicted AUC0-24h and maximum plasma concentration (Cmax) for severe renal impairment were 60% and 40% higher, respectively, than those of a typical individual with normal renal function. However, this difference is not considered to be clinically relevant given the relatively flat exposure-safety relationship over a 5-fold difference in exposure.
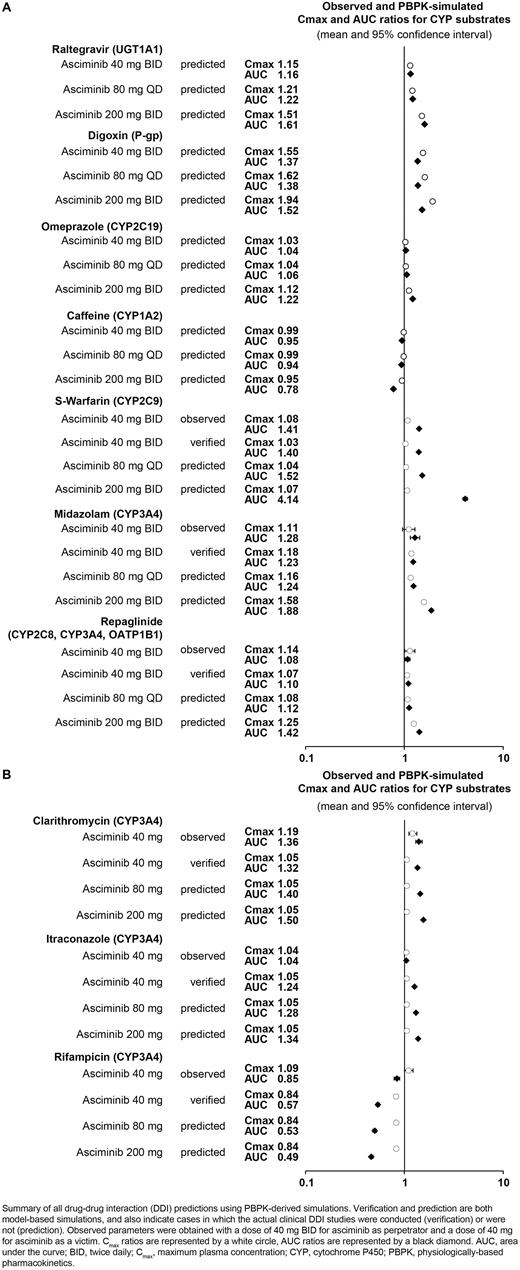
The PBPK model predicted no drug interaction that would significantly impact the PK of asciminib (as a victim) or of other substrates (as a perpetrator). Overall, asciminib exposure was weakly affected by interactions with inducers and inhibitors as a victim (Figure 1B) and was predicted to be a weak inhibitor of CYP3A4 and P-gp at all tested doses and a weak to moderate inhibitor of CYP2C9 as a perpetrator (Figure 1A).
Conclusions: Hepatic or renal impairment does not have a clinically significant impact on the PK of asciminib. Hence, dose adaptations for patients with hepatic or renal impairment are not warranted. Overall, asciminib has a manageable DDI risk at all recommended doses.
Sponsor: Novartis.
Disclosures
Hoch:Novartis: Current Employment, Current equity holder in private company. Huth:Novartis: Current Employment. Loisios Konstantinidis:Novartis: Current Employment. Xu:Novartis: Current Employment. Ho:Novartis: Current Employment. Combes:Novartis: Current Employment, Current equity holder in private company. Hourcade-Potelleret:Novartis: Current Employment, Current equity holder in private company. Einolf:Novartis: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal